Chronisch
myeloproliferative
Erkrankungen
Dr. Sormann Siegfried
Essentielle Thrombozythaemie
Essential thrombocythemia (ET) is one of the Philadelphia-negative classicalmyeloproliferativeneoplasms(MPNs).
Treiber Muatationen: JAK2, calreticulin Exon9, MPL
Thrombose, Blutungen, sek.vWJ Syndrom, post ETH-Myelofibrose
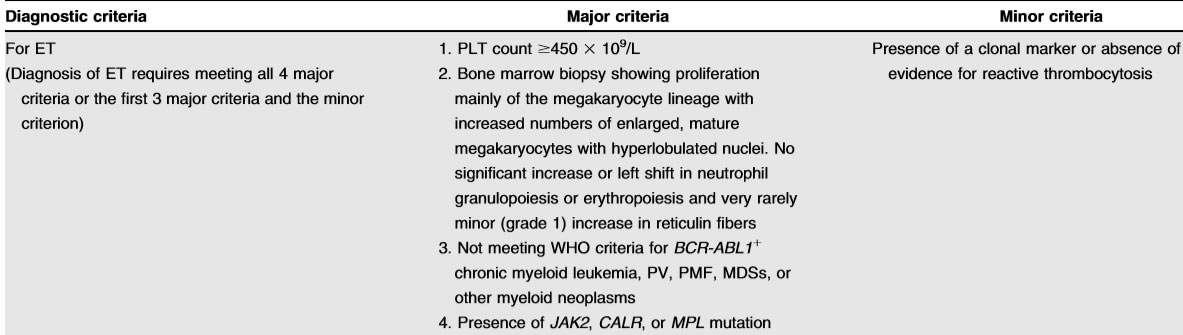
Definition
- platelet count of≥450×10(9)/L
- one of driver mutations: JAK2,CALR, andMPL
- exclusion of other causes of thrombocytosis (reactive and clonal)
- bonemarrow morphologic assessment, especially for distin-guishing ET from prefibrotic PMF and“masked”PV

Differentialdiagnose
Vorgangsweise
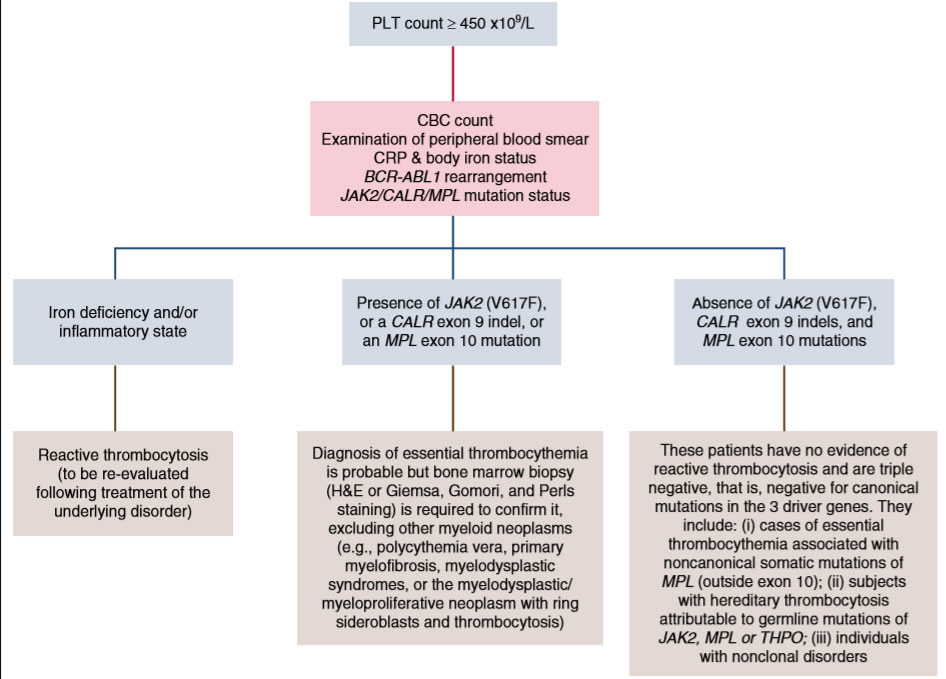
- Anamnese mit Erfassung der cardivaskulären Risikofaktoren - Thrombose
- CRP - Eisenstatus im Labor - reaktive Thrombozytose
- sek vWJ Syndrom bei hoher Thrombozytenzahl
- Oberbauchsono - Milzgröße - PMF - Jamshidi
- Treibermutationen - JAK2,Calreticulin,MPL
- Risikoscore IPSET, klassisch
- Knochenmarkpunktion bei jungen Patienten, fortgeschrittener Erkrankung,Splenomegalie (PMF), NGS zur Erfassung modifizierender Mutationen
Calreticulin Mutation
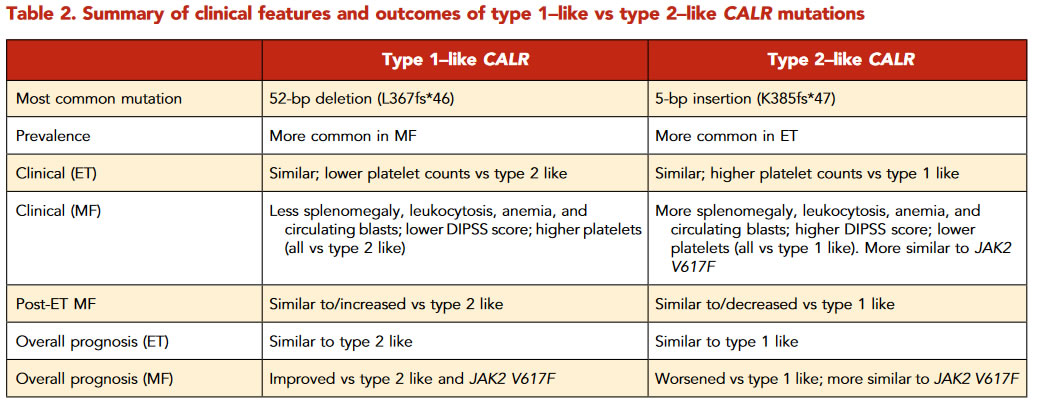
Calreticulin (CALR) ist ein Protein, das Teil eines Proteinkomplexes im Lumen des Endoplasmatischen Retikulums (ER) der Eukaryoten ist. Calreticulin fungiert zusammen mit Calnexin während der Faltung von Glykoproteinen im endoplasmatischen Retikulum als Chaperon. Dazu bindet es an Glykoproteine mit einem endständigen Glucoserest, der das Protein als noch nicht korrekt gefaltet kennzeichnet, und schützt es so vor Einflüssen aus der Umgebung, damit es sich richtig falten kann. Neben der Qualitätskontrolle der korrekten Glycoprotein-Faltung und der Sicherstellung der Calcium-Homöostase im ER hat CALR zahlreiche Funktionen außerhalb des ER, so im Zytoplasma, an der Zelloberfläche und auch in der extrazellulären Matrix, und beeinflusst dabei Zellproliferation, Apoptose, Phagozytose und die Immunreaktion. CALR kann auch den JAK-STAT-Signalweg in hämatopoetischen Zellen aktivieren.
presence of recurrent mutations inCALRin 70% to 80%of ET and PMF patients without aJAK2orMPLmutation.These mutations consist of insertions and/or deletions in exon 9,resulting in the generation of a novel mutant-specific positivelycharged amino acid sequence in the C terminus.
Compared withJAK2-mutant ET patients,CALR-mutant ET patients tend to be younger with lowerhemoglobin, decreased leukocytosis, and higher plateletcounts, and there is a higher male preponderance.There is robust evidence indicatingimproved thrombosis-free survival inCALR-mutant ET, with atwofold decreased risk for venous and arterial thrombosiscompared withJAK2 V617Fpatients
The riskof transformation to post-ET myelofibrosis (MF) seems to besimilar betweenCALR-mutant andJAK2-mutant patients
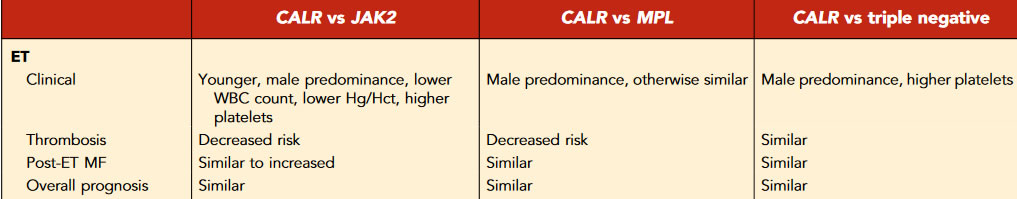
0TheJAK2 V617Fmutation confers higherthrombotic risk in the International Prognostic Score for EssentialThrombocythemia system.61In certain youngCALR-mutant ETpatients, thrombotic risk may be sufficiently low such that thesepatients can be managed with observation alone, without theaddition of aspirin.
 |
modifying Mutationen
- are found in ~53% of patients with ET
- TET2 (16%)
- ASXL1 (11%)
- DNMT3A (6%)
- SF3B1 (5%)
- SH2B3, SF3B1, U2AF1, TP53, IDH2, and EZH2 mutations as risk factors for overall, myelofibrosis-free or leukemiafree survival
- at least one of these mutations was seen in ~15% of the patients and median survival of patients with and without adverse mutations were 9 and 22 years, respectively
Risiken
vascular complication (15-year cumulative risk of thrombosis ranging from10%to25% depending on the molecular subtype, higher in JAK2-mutant than in CALR-mutant ET
post-PV Myelofibrose
selten Transformation in sek. AML
Score
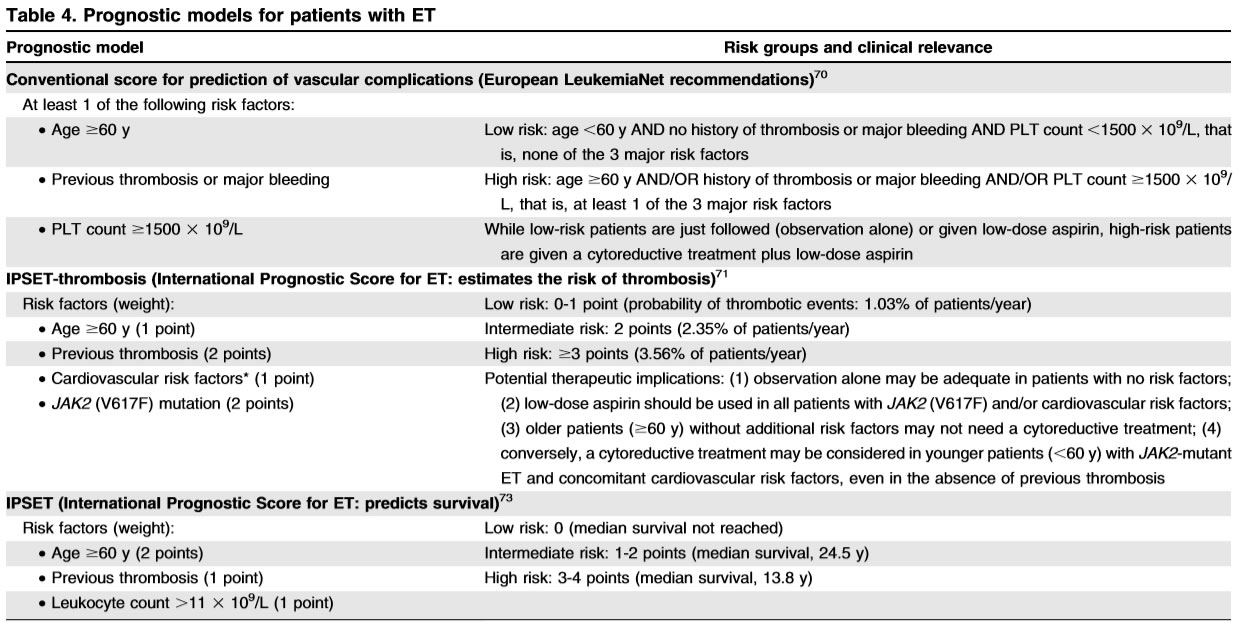
IPSET
The International Prognostic Score for Essential Thrombocythemia (IPSET)is based on age>60years,white blood cell(WBC)count >11 000, and history of thrombosis
Current treatment in ET is primarily indicated for the purposes of preventing thrombotic complications
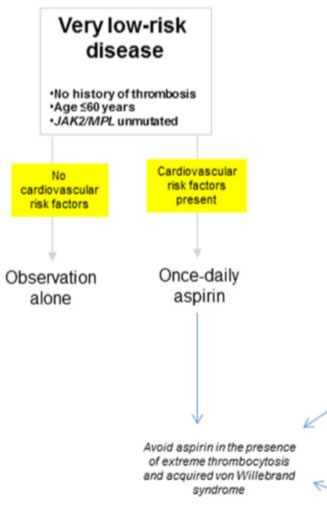
- very low-risk group
is defined by the absence of all three independent risk factors for thrombosis, including history of thrombosis, JAK2/MPL mutations, and advanced age - low-risk group
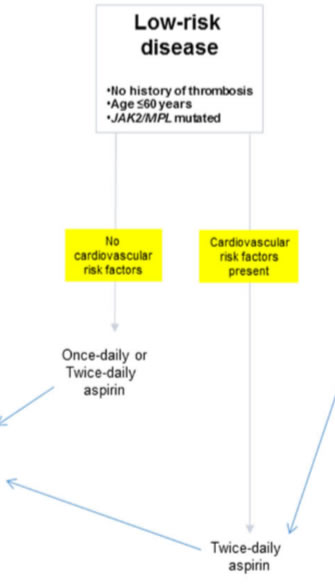
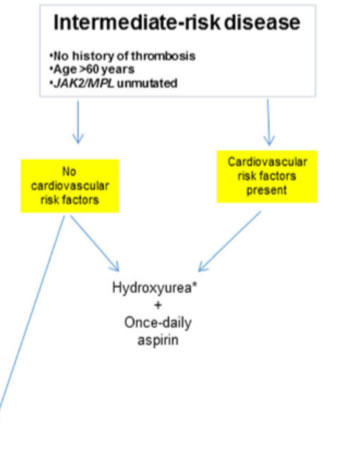
JAK2/MPL mutations in otherwise younger patients without history of thrombosis; - intermediate risk
JAK2/MPL unmutated older patients without thrombosis history; - high risk
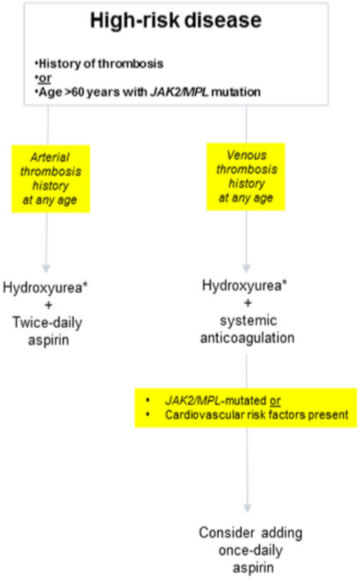
either presence of thrombosis history or presence of JAK2/MPL mutation in an older patient
Low risk
age < 60
no history of thrombosis or major bleeding
PLT count<1500
Therapie
JAK2 (V617F)-mutant ET • Low-dose aspirin
CALR-mutant ET, MPL-mutant ET and triple-negative patients • Low-dose aspirin* in patients with at least 1 concomitant cardiovascular risk factor† and/or with microvascular symptoms • Observation alone or low-dose aspirin* in patients without concomitant cardiovascular risk factors† and without microvascular symptoms
high risk
age > 60
history of thrombosis and/or major bleeding
PLT count>1500
Low-dose aspirin* 1 cytoreductive therapy
†Cardiovascular risk factors include hypertension, diabetes, dyslipidemia, and active tobacco use.
Therapie
 |
 |
 |
 |
Vorgangsweise
- very low risk
aspirin therapy in “very low-risk” ET should not be automatic, especially considering the fact that a substantial proportion of such patients display acquired von Willebrand syndrome.value of aspirin therapy in reducing the risk of arterial thrombosis in low-risk ET associated with CV risk factors, but not otherwise - low risk
residual risk of thrombosis despite management according to traditional treatment guidelines. Therefore, it is reasonable to consider further optimization of aspirin therapy in such patients by following “twice-daily” rather than “once-daily” schedule, especially in the presence of CV risk factors. inadequacy of once-daily aspirin dosing for 24-h optimal suppression of thromboxane-A2 synthesis, in the presence of high platelet turnover, and demonstration of superior biological efficacy in ET with twicedaily dosing - intermediate risk
Recent studies have suggested that “advanced age,” by itself, was a weak risk factor for thrombosis. therapeutically relevant since it provides the option of avoiding cytoreductive therapy in JAK2/MPL unmutated older patients without history of thrombosis or CV risk factors.the annual risk of thrombosis in such patients was 1.44%, compared to 4.17% in the presence of both JAK2 mutations and CV risk factors (p=0.01), and was similar to that of “low-risk” patients (1.59–2.57%). Aspirin - high risk
Accordingly, hydroxyurea, combined with once-daily aspirin therapy34, remains the standard of care for contemporarily classified “high-risk” patients. we underscore the need to maximize anti-thrombotic activity, by shortening the aspirin dosing schedule to every 12h, for patients with history of arterial thrombosis, and securing long-term systemic anticoagulation, in patients with history of venous thrombosis. In addition, it is reasonable to continue with once-daily aspirin therapy, along with systemic anticoagulation, in patients who are at risk for arterial thrombosis. Pegylated IFN-α treatment in ET has been shown to be relatively safe and effective, and has been associated with both clinical (70–80%) and molecular (10–20%) remissions in some patients, especially in the presence of CALR mutations.
Anagrelidelatter study, patients receiving anagrelide experienced higher incidences of arterial thrombosis, bleeding complications, and fibrotic progression. more than a quarter of patients receiving anagrelide therapy become anemic while a lesser percentage experience renal insufficiency and cardiac complications including arrhythmia and cardiomyopathy. currently consider anagrelide therapy only after failure of all other drug options, including hydroxyurea, IFN-α, and busulfan
ThromboAss - A randomized, double-blind trial of three aspirin regimens to optimize antiplatelet therapy in essential thrombocythemia
low-dose aspirin is the recommended antithrombotic regimen, but accelerated platelet generation may reduce the duration of platelet cyclooxygenase (COX)-1 inhibition.
Serum thromboxane B2 (sTXB2), a validated biomarker of platelet COX-1 activity
Evaluable patients assigned to the bid and tid regimens showed substantially reduced inter individual variability and lower median values of sTXB2: 19.3[9.7-40], 4 [2.1-6.7], and 2.5[1.45.65] ng/ml in the od (n=85), bid (n=79) and tid (n=79) arms, respectively.
The antiplatelet response to low-dose aspirin can be markedly improved by shortening the dosing interval to 12 hours
Aspirin permanently acetylates platelet and megakaryocyte cyclooxygenase (COX)-1, Under physiological megakaryopoiesis, the irreversible nature of COX-1 inactivation in the bone marrow progenitors leads to a new platelet progeny with largely non-functioning enzyme throughout the 24-hour dosin
Under physiological shear stress conditions, the COX-2 isozyme of vascular endothelial cells largely drives PGI2 biosynthesis, which has clinically relevant vasorelaxant and platelet-inhibiting effects, as suggested by the cardiovascular toxicity of COX-2 inhibitors.19 Low-dose aspirin has been shown to have limited inhibitory effects on in vivo PGI2 biosynthesis, possibly because of differential rates of recovery of endothelial COX-2 vs. platelet COX-1 during the 24-hour dosing
| ThromboASS 100mg | JAK2+; oder cardiovaskuläre Risikofaktoren = hypertension, diabetes, dyslipidemia, and active tobacco use |
| PEGasys | vorzugsweise Pat. < 60; CMPE Klon wird reduziert, molekulares Ansprechen, LPS,TSH,Psyche - Depression |
| Besemri | nicht zugelassen - PRV |
| Hydroxyurea | erste Wahl bei cytoreduktiver Therapie, wichtig auch die Leukozytose zu reduzieren |
| Anagrelide | gutes Ansprechen, Gefahr cardiovaskulär, nicht unterlegen zu HU, keine Wirkung auf Leukozytose |
| Jakavi | gutes Ansprechen |
OAS
therapy in ET has not been shown to affect survival
On the other hand, neither abnormal karyotype (detected in ~7% of patients) nor driver mutational status in ET has been shown to affect overall or leukemia-free survival; however, JAK2/MPL-mutated patients are significantly more thrombosis prone while MPL-mutated cases might be at a higher risk for fibrotic progression