diffus
grosszelliges
Non Hodgkin Lymphom
Dr. Sormann Siegfried
the most commonsubtype of non-Hodgkinlymphoma(NHL), accounting for 30% to 40% of all newly diagnosed cases
Vorgangsweise
- Typenbestimmung - GCB / nonGCB
- Molekularpathologie/Immunhistochemischer Algorithmus - DoubleHit, TripleHit, DoubleExpressor
- R-IPI; NCCN-IPI, ZNS-IPI
- Therapie
- R-CHOP
- bei hohem ZNS-IPI: R-CHOP+MTX
- bei IPI high risk; DH; TH, DE und junger Patient R-DA-EPOCH
- Relaps
- R-DHAP + ASCT
- Polivy-R-Benda
- Revlimid-Rituximab
Immunhistochemie
CD19, CD20, CD22, CD79a, CD45, IgG, in 14% CD30 (gute Prognose)
Typen
Unterteilung in verschiedene prognostische Typen erfolgte initial durch GEP.
GCB germ center like
und non-GCB like: ABClike und unklassifizierbar 10-15%
GEP nicht anwendbar - daher Anwendung eines Immunhistochemischen Algorithmus z.B. nach Green
Molekularpathologie:
Genmutationen - FISH - Translokationen:
c-myc 8q24 - Transkriptionsfaktor - Proliferation und Differenzierung - schlechte Prognose
bcl2 18q21 - Antiapoptose
BCL6 3q27 - Trankriptionsrepressor
doubleHit: c-myc + bcl2 oder bcl6
tripleHit: c-myc + bcl2 + bcl6
ca. 6-14% der de nov Fälle
ProteinExpression:
double expressor : c-myc + bcl2 - intermediate Prognose; ca. 30% de novo
DHT / THT - eher bei GCB
double expressor - eher bei ABC
 |
- GCB PFS 75% nach 3 Jahren - R-CHOP
- ABC PFS 40% nach 3 ahren - R-CHOP
- 15% nicht klassifizierbar
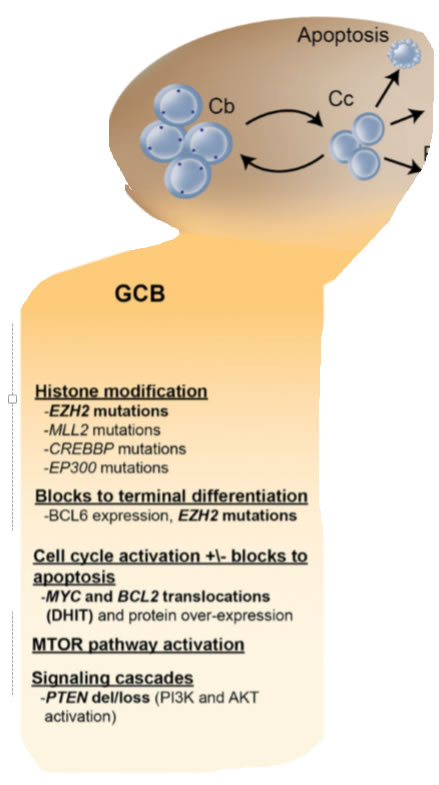
GCB - Genmuster, Immunhistochemie
- Ausgangspunkt Keimzentrum - Centroblasten
- Prognose gut 75% PFS nach 3 Jahren / RCHOP
- 30% to 40% t(14;18), 30% have c-rel amplification, 20% have mutations of the histonemethyltransferase EZH2,and 10% have a deletion of PTEN
- EZH2protein,leading to gain of function and the increased methylation of histone 3, which may promote lymphomagenesis by transcriptionally silencing key regulator genes
- Although deletion of PTEN,a tumorsuppressor gene affecting this pathway, has been noted in 10% of GCB DLBCL, the loss of PTEN expression by immunohistochemistry (IHC) was seen in 55% of GCB DLBCLcomparedwithonly 14% of non-GCBcases; The resultant constitutive activation of the PI3K/AKT/MTOR pathway in GCB DLBCL lacking PTEN
- In GCB DLBCL,overexpression of bcl2 is largely due to the presence of the t(14,18) translocation; Following treatment with R-CHOP, the negative prognostic impact of BCL2 overexpression appears to pertain only to GCB DLBCL
 |
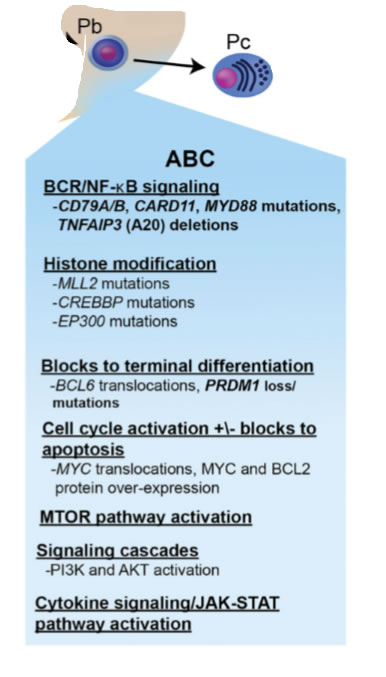
ABC - Genmuster, Immunhistochemie
- Ausgangspunkt plasmoblastische B Zelle nach Keimzentrum; just prior to germinal center exit, and therefore express genes that are frequently expressed in mature plasma cells
- Prognose schlechter 40% PFS nach 3 Jahren / RCHOP
- bcl2 überexpression other mechanisms, such as transcriptional upregulation and gene amplification, predominate
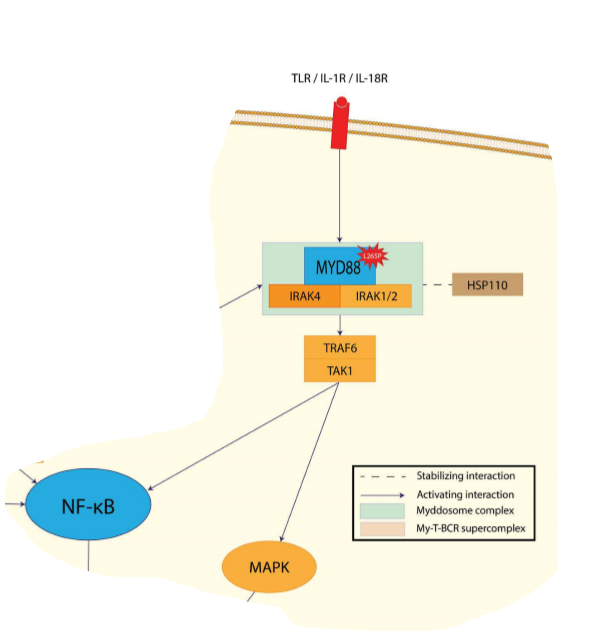
- hallmark of ABC DLBCL is the constitutive activation of the NF-kB signaling pathway, which promotes cell survival, proliferation, and inhibition of apoptosis; largely due to constitutive activation of the CBM signaling complex - (formed by CARD11, BCL10, and MALT1)
- 10% harbor activating mutations of CARD11,whereas in the remaining cases, chronic active B-cell receptor signaling engages the CBM pathway
- Chronic active B-cell receptor signaling is mediated through the B-cell receptor; harbors mutations in CD79A or CD79B)and downstream kinases, which include spleen tyrosine kinase (SYK), PI3K, Bruton tyrosine kinase (BTK), and protein kinase C b (PKCb);Recurring mutations in MYD88 have been observed in >30% upregulation of both the NF-kB and Janus kinase–signal transducer and activator of transcription pathways.
- the dual expression of these proteins myc bcl2 appears more common in the ABC subtype and may largely contribute to its inferior survival
 |
Myc und bcl2
- Increased expression of MYC protein promotes cellular growth and proliferation. Numerous studies have correlated the presence of a MYC rearrangement with a poorer outcome in DLBCL patients treated withR-CHOP
- that the impact of MYC is strongly influenced by BCL2. Concurrent MYC and BCL2 translocation,“double-hit”lymphoma,occurs in;5% ofcases of DLBCLand represents a treatment-refractory subgroup with a median survival of 8 months
5 Jahre PFS 27% und OAS 18%
Bio-Coral Studie R-DHAP+ASCT OAS 31% - Patients with dual expression of MYC and BCL2 protein, commonly referred to as dual-expressers, account for ;25% of DLBCL cases and have a significantly poorer outcome than patients who express only one or neither protein, with a 5-year PFS in the range of 25% following R-CHOP
- R-CHOP 6x (8x)
bis dato keine eindeutige Verbesserung durch zusätzliche Medika oder 14tägig - R-CHOEP oder R-DA-EPOCH
bei jungen Patienten mit DoubleHit, TripleHit oder Double Expressor; ebenfalls bei nonGCB like (ABC und nicht kalssifizierbar) - Relaps:
- R-DHAP + ASCT
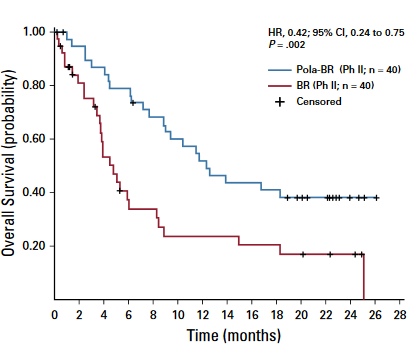
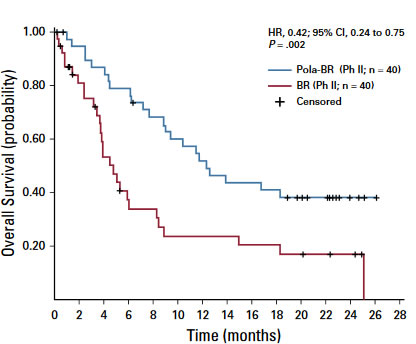
falls ASCT möglich bei Chemosensitivität - Polivy(Polatuzumab) + Rituximab + Bendamustin oder Gazyvaro + Benda
falls ASCT möglich bei Chemosensitivität - Polatuzumab vedotin - CD79b - microtubulus inhibitor
- CD79b is a signalingcomponent of the B-cell receptor located on normal B cellsand most mature B-cell malignancies, including.95% ofDLBC
- We report a phase Ib/II trial evaluating polatuzumab vedotincombined with bendamustine and obinutuzumab (pola-BG), and of pola-BR versus BR alone, in transplantation-ineligible R/R DLBCL, including patients who experiencedtreatment failure with prior ASCT
- All patients received bendamustine 90 mg/m2in-travenously (IV) on days 2 and 3 of cycle 1 and then days 1and 2 of subsequent cycles, and either rituximab IV(375 mg/m2on day 1 of each cycle) or obinutuzumab IV(1,000 mg on days 1, 8, and 15 of cycle 1 and day 1 ofsubsequent cycles). Those treated with polatuzumabvedotin received 1.8 mg/kg IV on day 2 of cycle 1 and day 1of subsequent cycles. Patients were treated for up to six 21-day cycles
- Overall incidence of PN was 43.6% (17/39) in pola-BRpatients (11 grade 1; 6 grade 2), with resolution in 10patients and improvement in 1 patient at clinical cutoff. PNwas the only reason for polatuzumab vedotin dose re-duction
- In the randomly assigned cohort, improvedoutcome with pola-BR was observed in both ABC and GCB
- L-MIND Studie - CD19 Ak
in Kombination mit Lenalidomid
weitere studie CD19 + Bendamustin versus RBenda - Blinatumomab + Pembrolizumab
bispezifisch CD3+CD19
PD1 Inhibitor
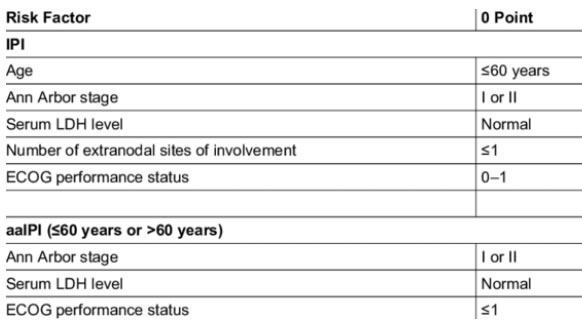
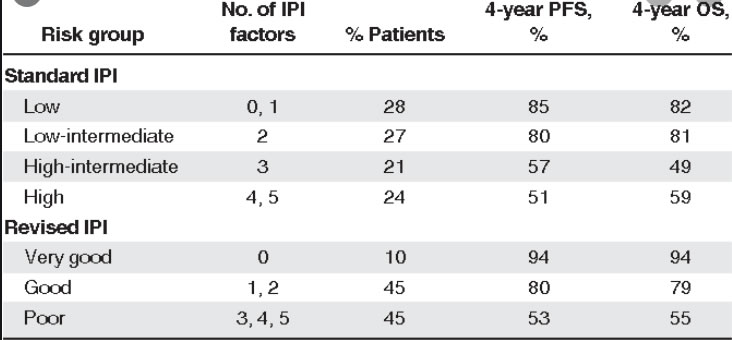
IPI / NCC-IPI
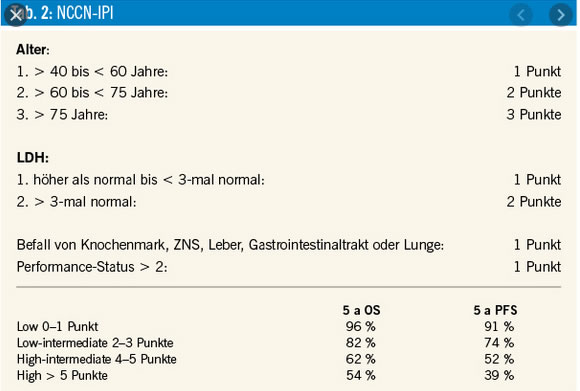 |
Compared with the IPI, the NCCN-IPI was better able to discriminate a high-risk group with 5-year OS of 33%, although it represented only 8% to 14% of the patients
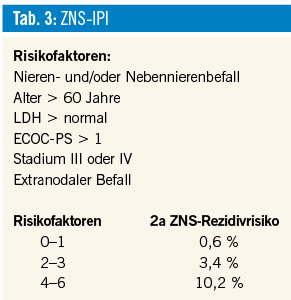
ZNS- IPI
 |
 |
OAS 3 Jahre
0-1 91%
2 81%
3 65%
4 59%
 |
Therapie
The rituximab with CHOP over age 60 (RICOVER-60) trial also confirmed that 6 cycles of therapy was sufficient for patients with advanced stage disease.
Approximately 60% of patients will be cured, with more favorable outcomes seen in patients with limitedstagedisease.Patients who remain event-free at24months have recently been shown to have an OS comparable to the general population, further highlighting the importance of optimizing front-line therapy
R-CHOP -Intensifikation:
O-CHOP
DA-EPOCH - CALGB/Alliance kein Unterschied EFS OAS zu R-CHOP / aber wenig DH/TH Patienten
R-CHOP 14
keine Verbesserungen
non-GCB like: Zugabe von Ibrutinib - Lenalidomid - Bortezomib
ABClike ist bcl2 aktiviert und wird durch Ibrutinib und Bortezomib inhibiert.
R-CHOP+Ibrutinib wurde gestoppt - da PFS endpont nicht erreicht wurde
R-CHOP + Bortezomib - initial bessere PFS konnte nicht bestätigt werden.
R-CHOP + Lenalidomid - ähnliches PFS für GCB und nonGCB - ORR 98% - ROBUST- Studie
ROBUST (CC-5013-DLC-002) is a randomized, double-blind, global, Phase III study of oral lenalidomide (15 mg, days 1–14) plus R-CHOP21 × 6 versus placebo-R-CHOP21 × 6 in patients with previously untreated ABC-type DLBCL
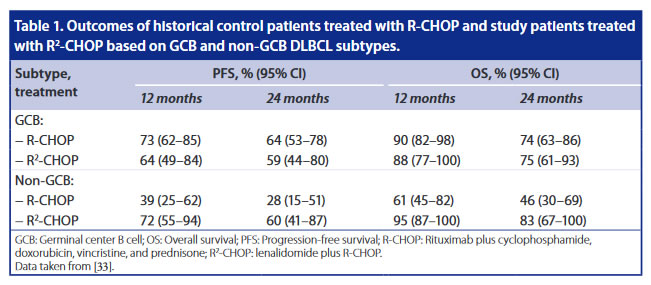 |
Overall, the ROBUST study did not meet the primary endpoint of PFS for R 2 -CHOP vs placebo/R-CHOP in previously untreated patients with ABC-DLBCL, although a positive trend favor- ing R 2 -CHOP has been observed in advanced stage and higher risk patients. The safety profile of R 2 -CHOP was consistent with those of individual medicines, and no new safety signals were identified with the combination
Vorgangsweise
 |
 |
